In 2022, the Office of Science and Technology Policy (OSTP) published guidance on public access to research. The OSTP Memo --also called Nelson Memo-- affected all Federal Funding Agencies, establishing provisions for the sharing of data and scholarly publications of research funded by those agencies, and the use of persistent identifiers (ORCID, ROR, DOIs, etc.) with implementation in 2025/2026.
Beginning in 2024, Federal Agencies started to release new policies and guidelines on Publication and Data Sharing to comply with the OSTP Memo. Requirements and starting date of compliance vary between agencies. See the SPARC Guide for Authors Complying with U.S. Federal Agency Public Access and Publisher Policies, or check below for more information. For data requirements, the University of Rochester Libraries’ Data Services team is available for assistance; for publication requirements, the RCL Scholarly Communications team can assist with any inquiries.
For most federal agencies, Author Accepted Manuscripts (AAM) of peer-reviewed publications related to federally funded research must be deposited on an agency-designated or recommended repository, without embargo, at the time of publication.

Some key points:
- Public access is different than open access.
- Open access resources are publicly available (free to read) and have an open access license modifying their copyright and returning some rights to the readers.
- Public access resources are publicly available (free to read) without any additional provisions regarding copyright or licensing. Public access resources can be under full copyright (author or publisher).
- The AAM is the final version post-peer review but before publisher formatting. This is the required version for submission.
- The Final Published Article is the journal’s formatted version. While submission of the Final Published Article is an option (for open access articles or journals with agreements with federal agencies), it is not required for compliance.
- Compliance is free; researchers are not required to pay article processing charges (APC) to meet the new policy.
Recommendations for authors
There is currently a disconnect between federal public policies and many publishers' policies, with the latter still requiring a 6 to 12-month embargo for the Author Accepted Manuscript to be deposited. That leads authors to sign contracts with publishers that may contradict the federal mandate. Check the Open Policy Finder for journal/publisher policies, or contact Scholarly Publishing Services to discuss your options
Until that changes, the recommendation on the NIH Supplementary Guidance is for authors to add to the submission letters of any future publications some suggested language to let the journal editors know that the authors have funder requirements they have to meet.
Example: "This manuscript is the result of funding in whole or in part by the [Federal Agency]. It is subject to the [Federal Agency]'s public access policy. Through acceptance of this federal funding, the [Federal Agency] has been given the right to make this manuscript publicly available in [recommended repository], upon the Official Date of Publication as defined by the [Federal Agency]."
Compliance workflow
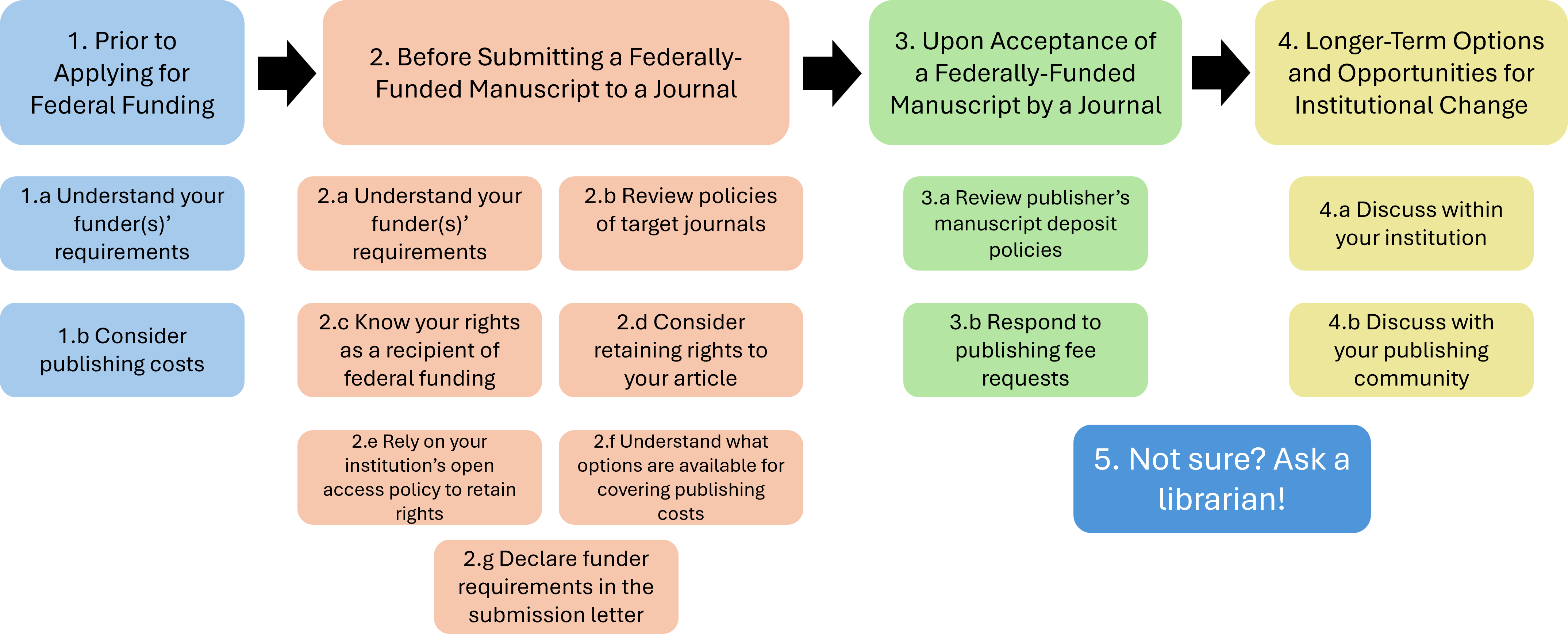
- Prior to Applying for Federal Funding
- Understand your funder(s)’ requirements
- Consider publishing costs
- Before Submitting a Federally-Funded Manuscript to a Journal
- Understand your funder(s)’ requirements
- Review policies of target journals
- Know your rights as a recipient of federal funding
- Consider retaining rights to your article
- Rely on your institution’s open access policy to retain rights
- Understand what options are available for covering publishing costs
- Declare funder requirements in the submission letter
- Upon Acceptance of a Federally-Funded Manuscript by a Journal
- Review publisher’s manuscript deposit policies
- Respond to publishing fee requests
- Longer-Term Options and Opportunities for Institutional Change
- Discuss within your institution
- Discuss with your publishing community
- Not sure? Ask a librarian! Contact Scholarly Publishing Services
Federal Purpose License
The Federal Purpose License (sometimes called the “government use license”) is a nonexclusive, irrevocable, royalty-free license that allows the government to reproduce, publish, or use work funded by government grants “for federal purposes.” It also gives the agency the authority to authorize others to use the work. Federal agencies can rely on the Federal Purpose License for their requirements.
1) Ensures institutions can comply with federal policies regardless of what an author agrees to in subsequent licensing agreements.
2) Spares authors from having to negotiate with publishers to retain the rights needed to comply with federal policies.
Learn more here: The Federal Purpose License – What Campuses Need to Know
Rivers Campus Libraries have signed the Right to Deposit Statement: “Thus, we, as signatories to this statement, support reliance on the Federal purpose license by federal agencies for implementation of the Nelson memo.”
Resources
- Federal Grants and Institutional IP Policies, from the Authors Alliance and SPARC
- SPARC Agency Publication and Data Sharing Policy Resource
- The NIH Public Access Policy: Q&A for Authors, from the Authors Alliance
- Updated NIH and Publisher Guidance: What Authors Need to Know about NIH’s Public Access Policy, from the Authors Alliance
- Guidance on the Revised NIH Public Access Policy, Effective July 2025, from the University of California

